Personalized Medicine The Future of Tailored Healthcare
Personalized medicine represents a transformative approach in healthcare, focusing on tailoring medical treatment to the individual characteristics of each patient. By leveraging genetic profiles and advanced diagnostics, it promises more effective and efficient care. This article delves into the intricacies of personalized medicine, its development, and its potential to redefine healthcare paradigms.
The Genesis of Personalized Medicine
The concept of tailoring healthcare to individual genetic profiles is rooted in centuries of medical evolution, yet its modern form emerged from groundbreaking scientific discoveries. While Hippocrates first suggested that health was influenced by personal traits, it wasn’t until the 20th century that genetics provided the framework for true personalization. The discovery of DNA’s double helix structure by Watson and Crick in 1953 laid the foundation, but the real turning point came with the Human Genome Project (HGP), completed in 2003. This monumental effort mapped the entire human genome, revealing the genetic variations that influence disease susceptibility and drug response.
Before the HGP, medicine relied on broad population-based treatments, often with unpredictable outcomes. The project’s success enabled researchers to identify single nucleotide polymorphisms (SNPs) and other genetic markers linked to diseases like cancer, diabetes, and cardiovascular disorders. Pharmacogenomics, a field born from these insights, demonstrated how genetic differences affect drug metabolism—explaining why some patients respond well to a treatment while others experience adverse effects.
Key milestones accelerated personalized medicine’s adoption:
- 1970s-1980s: Development of DNA sequencing techniques, notably Sanger sequencing, allowed precise genetic analysis.
- 1990s: The advent of polymerase chain reaction (PCR) made genetic testing faster and more accessible.
- 2000s: Next-generation sequencing (NGS) reduced costs and time, enabling widespread genomic profiling.
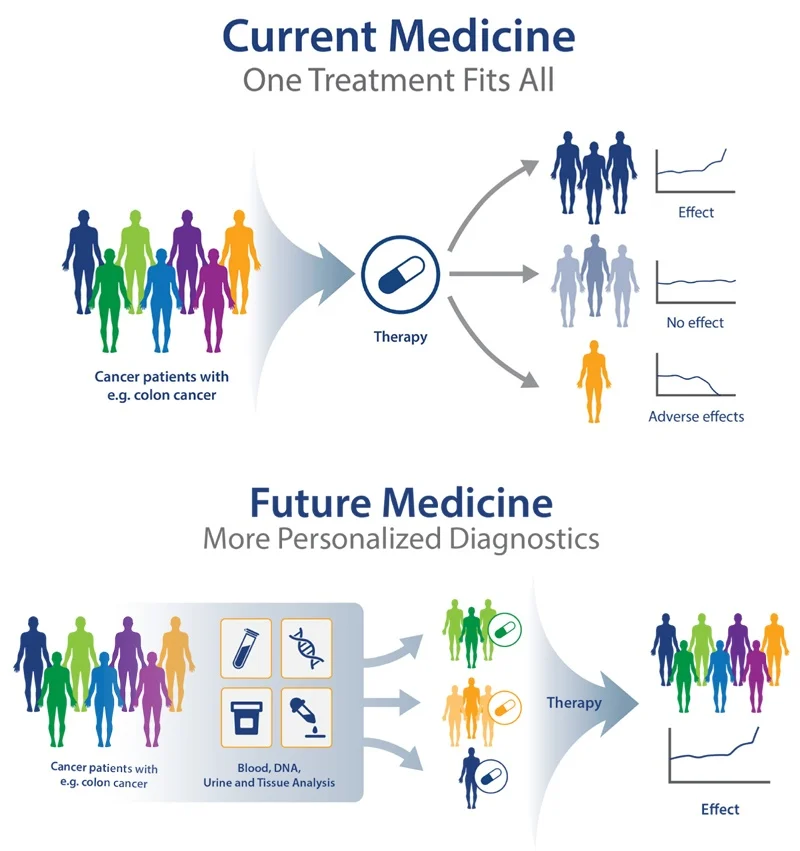
These advancements transformed medicine from a reactive to a proactive discipline. Today, genetic profiling informs everything from cancer therapies to prenatal screenings, ensuring treatments align with a patient’s unique biology. As we move forward, the integration of AI and big data promises even deeper personalization, setting the stage for the next chapter: Understanding Genetic Profiling.
Understanding Genetic Profiling
Genetic profiling lies at the core of personalized medicine, enabling healthcare providers to tailor treatments based on an individual’s unique DNA blueprint. At its essence, genetic profiling involves analyzing a person’s genome to identify variations—such as single nucleotide polymorphisms (SNPs), insertions, deletions, or copy number variations—that influence disease susceptibility, drug metabolism, and therapeutic response. Advances in sequencing technologies, particularly next-generation sequencing (NGS), have made it possible to decode entire genomes rapidly and cost-effectively, unlocking unprecedented precision in medical decision-making.
The process begins with extracting DNA from a patient’s blood, saliva, or tissue sample. This genetic material is then sequenced, generating vast datasets that are analyzed using bioinformatics tools to pinpoint clinically relevant mutations. For example, identifying a BRCA1 or BRCA2 mutation can guide preventive strategies for breast cancer, while detecting variations in the CYP2C19 gene helps predict a patient’s response to blood thinners like clopidogrel.
Beyond disease risk assessment, genetic profiling enables the customization of therapies by matching patients with drugs most likely to be effective for their genetic makeup. This approach minimizes trial-and-error prescribing, reducing adverse effects and improving outcomes. Additionally, pharmacogenomic data—integrated with electronic health records—empowers clinicians to make real-time, evidence-based adjustments to treatment plans.
However, challenges remain, including interpreting the clinical significance of rare variants and ensuring equitable access to genetic testing. Ethical considerations, such as data privacy and the potential for genetic discrimination, also demand careful attention. As the field evolves, integrating artificial intelligence and machine learning will further refine genetic interpretations, solidifying personalized medicine as the future of healthcare.
The Role of Pharmacogenomics
Pharmacogenomics stands at the forefront of personalized medicine, bridging the gap between genetic profiling and tailored therapeutic strategies. By analyzing how an individual’s genetic makeup influences their response to medications, this field empowers clinicians to optimize drug selection, dosage, and efficacy while minimizing adverse effects. Unlike traditional trial-and-error approaches, pharmacogenomics leverages single nucleotide polymorphisms (SNPs) and other genetic variants to predict drug metabolism, transport, and target interactions.
Key mechanisms include:
- Drug Metabolism: Enzymes like CYP2D6 and CYP2C19 metabolize many medications. Genetic variations in these enzymes can classify patients as poor, intermediate, extensive, or ultra-rapid metabolizers, drastically altering drug effectiveness or toxicity.
- Drug Transport: Proteins such as P-glycoprotein, encoded by the ABCB1 gene, influence drug absorption and distribution. Mutations here can affect concentrations of drugs like digoxin or chemotherapy agents.
- Drug Targets: Genetic differences in receptors (e.g., VKORC1 for warfarin) or signaling pathways can determine whether a drug will bind effectively or cause unintended reactions.
The clinical impact is profound. For instance, testing for HLA-B*57:01 prevents life-threatening hypersensitivity to abacavir in HIV patients, while TPMT genotyping guides thiopurine dosing in leukemia to avoid severe myelosuppression. However, challenges remain, including the need for widespread genetic testing infrastructure and clinician education to interpret results.
As diagnostic tools evolve (discussed in the next chapter), pharmacogenomics will integrate deeper into routine care, transforming prescriptions from generalized guesses into precise, data-driven decisions. This shift not only enhances patient outcomes but also reduces healthcare costs by avoiding ineffective treatments and preventable complications.
Diagnostic Tools and Technologies
Advances in diagnostic tools and technologies are the backbone of personalized medicine, enabling clinicians to tailor treatments based on an individual’s genetic and molecular profile. Unlike pharmacogenomics, which focuses on drug-gene interactions, these tools provide a broader diagnostic foundation, uncovering disease mechanisms at an unprecedented resolution. Molecular diagnostics, for instance, leverages techniques like next-generation sequencing (NGS) and polymerase chain reaction (PCR) to detect genetic mutations, biomarkers, and microbial pathogens with high accuracy. These methods allow for early disease detection, risk assessment, and monitoring of treatment efficacy, ensuring interventions are precisely aligned with a patient’s biology.
Imaging technologies have also evolved to support personalized care. Advanced modalities such as functional MRI (fMRI), positron emission tomography (PET), and molecular imaging provide real-time insights into cellular and metabolic processes. For example, PET scans combined with radiopharmaceuticals can visualize tumor-specific markers, guiding oncologists in selecting targeted therapies. Similarly, AI-enhanced imaging algorithms analyze vast datasets to predict disease progression, further refining treatment plans.
Emerging tools like liquid biopsies and wearable biosensors are pushing boundaries even further. Liquid biopsies detect circulating tumor DNA (ctDNA) from a simple blood draw, offering a non-invasive alternative to tissue biopsies for cancer monitoring. Wearables, equipped with genomic and proteomic sensors, continuously track physiological data, enabling dynamic adjustments to therapy.
The integration of these technologies into clinical workflows is transforming healthcare from reactive to proactive. By decoding the molecular and genetic underpinnings of disease, clinicians can move beyond one-size-fits-all approaches, ensuring each patient receives the most effective, least invasive care possible. This shift underscores the synergy between diagnostics and treatment, bridging the gap between pharmacogenomics and the broader precision medicine landscape.
Precision vs Personalized Medicine
Precision medicine and personalized medicine are often used interchangeably, but they represent distinct yet overlapping approaches in modern healthcare. Precision medicine focuses on tailoring treatments based on subgroup characteristics, such as genetic markers or molecular profiles, to improve efficacy for specific patient populations. In contrast, personalized medicine goes further by considering individual variability—including genetics, lifestyle, and environmental factors—to craft unique treatment plans.
A key distinction lies in scalability. Precision medicine often relies on biomarkers or genetic signatures shared among groups, enabling standardized therapies for subsets of patients. For example, HER2-positive breast cancer patients receive targeted therapies like trastuzumab, a precision approach. Personalized medicine, however, integrates broader data—such as epigenetics, microbiome composition, or even patient preferences—to design truly individualized care.
Misconceptions arise when assuming precision medicine always equates to personalization. While all personalized medicine is precise, not all precision medicine is personalized. For instance, pharmacogenomics—a pillar of precision medicine—uses genetic data to predict drug responses but may not account for a patient’s unique lifestyle or comorbidities. True personalization demands a holistic view, merging advanced diagnostics (as discussed earlier) with continuous monitoring and adaptive strategies.
The overlap emerges in their shared reliance on cutting-edge technologies, like next-generation sequencing or AI-driven analytics, to decode biological complexity. Both aim to move beyond the “one-size-fits-all” model, yet personalized medicine’s ambition is broader, striving for hyper-customized care. As the following chapter will illustrate through case studies, the integration of these approaches is already transforming outcomes, proving that the future of healthcare lies in balancing precision with true personalization.
Case Studies in Personalized Treatment
Personalized medicine has already demonstrated its transformative potential through real-world applications, where genetic profiling has led to breakthroughs in patient care. One notable example is the treatment of chronic myeloid leukemia (CML). Before the advent of targeted therapies, CML had limited treatment options, often requiring aggressive chemotherapy. The discovery of the BCR-ABL fusion gene, a genetic hallmark of CML, paved the way for imatinib, a tyrosine kinase inhibitor designed to specifically block the abnormal protein produced by this mutation. Patients with this genetic marker now experience significantly improved survival rates, with many achieving long-term remission—a stark contrast to previous outcomes.
Another compelling case is cystic fibrosis (CF), a genetic disorder caused by mutations in the CFTR gene. Historically, treatment focused on managing symptoms rather than addressing the root cause. However, the development of ivacaftor, a drug targeting specific CFTR mutations, has revolutionized care. Patients with the G551D mutation, for instance, show marked improvements in lung function and quality of life, underscoring how genetic insights can drive precision therapies.
In oncology, HER2-positive breast cancer exemplifies the power of personalized medicine. The identification of HER2 gene amplification allowed for the development of trastuzumab, a monoclonal antibody that selectively targets HER2-overexpressing tumors. This approach has drastically reduced recurrence rates and improved survival, proving that molecular diagnostics can redefine treatment paradigms.
These cases highlight a critical shift: from one-size-fits-all medicine to therapies tailored to individual genetic profiles. While challenges remain—as explored in the next chapter—the successes of personalized medicine underscore its potential to deliver more effective, patient-specific care. The integration of genetic data into clinical practice is no longer theoretical; it is a reality transforming lives.
Challenges in Implementation
While personalized medicine has demonstrated remarkable success in tailoring treatments, as highlighted in the previous chapter, its widespread adoption faces significant hurdles. Ethical, financial, and technological challenges create barriers that must be addressed to fully integrate genetic profiling into mainstream healthcare.
Ethical dilemmas arise around data privacy and consent. Genetic information is highly sensitive, and breaches could lead to discrimination in employment or insurance. Additionally, unequal access to personalized therapies exacerbates healthcare disparities, favoring those in wealthier nations or with better insurance coverage. The question of who owns genetic data—patients, researchers, or corporations—further complicates ethical frameworks.
Financial constraints present another major obstacle. Developing and administering genetic tests and targeted therapies is costly, straining healthcare budgets. While some treatments reduce long-term expenses by avoiding trial-and-error prescribing, the upfront investment is prohibitive for many institutions. Reimbursement models lag behind innovation, leaving patients and providers uncertain about coverage for cutting-edge genomic therapies.
Technological limitations also hinder progress. Despite advances, sequencing and analyzing vast genomic datasets require sophisticated infrastructure and expertise. Many healthcare systems lack the computational power or trained personnel to interpret genetic data accurately. Interoperability issues between electronic health records and genomic databases further slow integration, limiting real-time clinical application.
Overcoming these challenges demands collaboration among policymakers, researchers, and healthcare providers. Standardizing data protocols, expanding funding for genomic research, and establishing clear ethical guidelines will be critical to unlocking personalized medicine’s full potential—a transition that could reshape healthcare economics, as explored in the next chapter.
The Economic Impact of Personalized Healthcare
The shift toward personalized medicine carries significant economic implications, reshaping healthcare spending in both promising and challenging ways. By leveraging genetic profiling to tailor treatments, healthcare systems can reduce inefficiencies, minimize trial-and-error prescribing, and avoid costly adverse drug reactions. Studies suggest that pharmacogenomic testing alone could save billions annually by optimizing drug selection and dosage, particularly in chronic conditions like cardiovascular disease and mental health disorders.
However, the upfront costs of implementing personalized medicine are substantial. Genetic sequencing, data storage, and specialized infrastructure require significant investment. While prices for genome sequencing have dropped dramatically, integrating these technologies into routine care demands ongoing funding for training, software, and interoperability with existing systems. Payers—whether governments, insurers, or patients—must weigh these initial expenses against long-term savings, which may take years to materialize.
Another economic consideration is the potential for healthcare disparity. High-cost genetic therapies and diagnostics risk widening gaps in access, particularly in underfunded health systems. Conversely, targeted treatments could lower overall costs by reducing hospitalizations and ineffective interventions. For example, oncology treatments guided by tumor genomics often yield higher success rates, shortening hospital stays and improving outcomes.
The financial burden also extends to research and development. Drugmakers face pressure to create niche therapies for smaller patient populations, raising questions about pricing sustainability. While personalized therapies command premium prices, their precision may justify the cost if they replace broader, less effective treatments. Policymakers must balance innovation incentives with affordability to ensure equitable adoption.
As the field evolves, economic models must adapt to account for these dynamics, ensuring that personalized medicine delivers value without exacerbating systemic inequities. The next challenge lies in addressing the ethical and privacy concerns tied to this data-driven approach.
Ethical Considerations and Patient Privacy
As personalized medicine advances, the ethical dilemmas and privacy concerns surrounding genetic data usage become increasingly critical. The ability to tailor treatments based on an individual’s genetic profile offers immense potential, but it also raises questions about consent, data security, and equitable access. Who owns genetic data? How can it be protected from misuse? These are just a few of the pressing issues that must be addressed to ensure the responsible growth of this field.
One major concern is the risk of genetic discrimination. Employers or insurers could misuse genetic information to deny opportunities or coverage based on predispositions to certain diseases. While laws like the Genetic Information Nondiscrimination Act (GINA) in the U.S. offer some protection, gaps remain, particularly in life insurance and other sectors. Patients may also hesitate to share their data if they fear it could be exploited, undermining the collective benefits of large-scale genetic research.
Data privacy is another significant challenge. Genetic information is uniquely identifiable and immutable, making breaches particularly damaging. Even anonymized data can sometimes be re-identified, raising concerns about how securely it is stored and shared. Companies and healthcare providers must implement robust encryption and strict access controls to prevent unauthorized use.
Additionally, ethical questions arise around informed consent. Many patients may not fully understand how their genetic data could be used in research or commercial applications. Transparent communication and dynamic consent models—where patients can update their preferences over time—are essential to maintaining trust.
Finally, there’s the issue of equity. If genetic testing and tailored treatments remain expensive, they could exacerbate healthcare disparities. Ensuring affordable access while safeguarding privacy will be key to realizing the full potential of personalized medicine without leaving vulnerable populations behind.
The Future of Personalized Medicine
The future of personalized medicine promises a paradigm shift in healthcare, driven by rapid advancements in genomics, artificial intelligence (AI), and machine learning (ML). As genetic profiling becomes more accessible and affordable, the integration of these technologies will enable hyper-personalized treatment plans that adapt in real-time to an individual’s evolving health data.
One of the most anticipated breakthroughs is the development of dynamic treatment algorithms. These systems will analyze a patient’s genetic makeup, lifestyle, and environmental factors to predict disease risks before symptoms appear. AI-powered platforms will then recommend preventive measures or early interventions, drastically reducing the burden of chronic illnesses. For example, ML models trained on vast genomic datasets could identify subtle genetic markers linked to drug resistance, allowing physicians to prescribe the most effective medications from the outset.
Another frontier is the use of CRISPR-based gene editing for personalized therapies. While still in experimental stages, advancements in precision editing could one day correct genetic mutations at the source, offering cures for conditions like sickle cell anemia or cystic fibrosis. AI will play a critical role here, optimizing gene-editing protocols to minimize off-target effects and maximize efficacy.
Wearable devices and continuous health monitoring will further enhance personalized medicine. Integrated with AI, these tools will provide real-time feedback on how a patient’s body responds to treatments, enabling dynamic adjustments. Imagine a smartwatch that detects early signs of a metabolic imbalance and automatically alerts a healthcare provider to modify a treatment plan.
The convergence of genomics, AI, and IoT will create a closed-loop healthcare ecosystem, where treatments are not just tailored but also self-optimizing. However, this future hinges on overcoming technical challenges, such as data interoperability and algorithmic bias, while maintaining the ethical safeguards discussed earlier. The potential is immense—personalized medicine could soon make one-size-fits-all healthcare a relic of the past.
Conclusions
Personalized medicine stands at the forefront of medical innovation, offering a future where treatments are as unique as the individuals receiving them. By harnessing genetic insights and cutting-edge technology, it paves the way for more precise, preventive, and participatory healthcare. The journey towards fully personalized medicine is complex but undeniably promising.



